Natural
Length Contraction Mechanism Due to Kinetic Energy
Paul Marmet
( Last checked 2023/08/21 - The estate of Paul Marmet
)
Abstract.
This
paper
shows
that
the
phenomena
usually
attributed
to relativity are a simple consequence of mass-energy
conservation. When atoms are accelerated, the increase of
kinetic energy increases the electron mass, which makes the Bohr
radius larger. This increase of radius produces a shift in
the atomic energy levels and also an increase of the physical
size of matter. Consequently, a moving atomic clock now
runs at a different rate. Quite naturally and without
Einstein's relativity, we see how the increase of size of the
Bohr radius and of macroscopic matter, are exactly equal to
Einstein's prediction. Einstein's theory of relativity
predicts length contraction, but does not explain how matter can
be physically contracted or why this phenomenon is not
reversible when the mass in the moving frame is accelerated back
to the original frame. Einstein's length contraction
implies that the Bohr atom gets smaller. However, quantum
mechanics shows that such a contraction of the Bohr radius
should increase the atomic energy levels. This consequence
of Einstein's predictions is contrary to observational facts,
which show that, at high velocity, the atomic energy levels
become smaller and the atomic clocks get slower. The
mechanism of dilation and contraction of matter is logically
explained here, using Newton physics and the fundamental
principles of quantum mechanics. Using the de Broglie
equation, we calculate the relationship between the Bohr radii
in different frames, which is responsible for the physical
change of length of matter, in agreement with all observational
data. Furthermore, just as for mass and energy units, we
show that the physical size of the Planck unit, needs to
increase g times with
velocity. Observations previously attributed to relativity can
now be explained logically. These results are also
compatible with a rational explanation of the advance of the
perihelion of Mercury around the Sun. We must conclude
that there exists no space-time distortion and the Einstein's
relativity principle cannot be valid. Everything is
naturally explained as a change of size of matter and a change
of clock rate. A corresponding solution also exists in the
case of gravitational energy as will be demonstrated in a future
paper. These phenomena, taking place in atoms are also
predictable in the nucleus of matter. For the same reason,
the lifetimes of radioactive nuclear states also changes
naturally with velocity and potential energy.
1- Introduction.
The problem of dilation and contraction of matter in
relativity could never be explained logically.
Einstein's relativity presents no physical rationalization
explaining why and how matter can dilate or contract.
That field of physics is impenetrable, because it is not
compatible with the existence of a physical reality,
independent of the observer's existence. Einstein's
theory has never been expressed unequivocally and the more
recent theoretical developments collapse into a deeper
mystery. Unfortunately, just as during the Middle Ages,
most scientists accept the idea that nature is not compatible
with conventional logic. Nowadays, most scientists
ignore or refuse to read papers implying an existence of
matter independent of the observer.
In
this
paper,
the
phenomenon
of
length
contraction
or dilation is explained logically without any of Einstein's
relativity hypotheses (1).
Everything is explained as a function of the physics of
Newton, Coulomb and de Broglie. Due to the increase of
kinetic energy and the application of the principle of
mass-energy conservation, we see that the Bohr radius
increases, so that the physical size of matter
increases. This dilation of matter is not a simple
mathematical transformation visible by only one observer in a
specific frame, it is a physical reality. Also, matter
shrinks back to its original length when the velocity is
reduced. The fundamental principles related to this
natural phenomenon have been explained previously (2).
The
fundamental
reason
for
which
the
Bohr
radius
increases when kinetic energy is given to the atom is
mechanical. In a few words, atoms in space are like
gyroscopes moving freely in space. The orbiting electron
around the nucleus represents the spinning wheel. When
the mass of the spinning wheel (here the orbiting electron
inside an atom) increases, the velocity of rotation decreases,
due to momentum conservation. Therefore in the Bohr
atom, when the electron mass increases, the electron velocity
decreases. Using Coulomb forces, it is well known that
the radius of the electron orbit is larger when the electron
velocity becomes slower. Consequently, the Bohr radius
becomes larger when the atom is moving faster. The
change of Bohr radius is the fundamental cause of dilation of
matter when atoms acquire kinetic energy. This mechanism
is calculated in detail in this paper.
In
agreement
with
observations,
we
show
also
that,
even if in fact the physical size of the atom is increasing,
the quantum structure of the moving atom is changing in such a
way that these changes of mass and length remain generally
undetectable to the moving observer. The esoteric
Einstein's hypothesis of space-time distortion is
unacceptable, because it is not compatible with a physical
reality independent of the observer. Contrary to most
papers in modern physics, we always refer here to a realistic
physical model. We question the physical interpretation
of equations. There must be no mathematical model in
physics, without being logically supported by a physical
model. One of the most important errors in Einstein's
relativity is that, the inevitable change of sizes of some
moving reference units (i.e. the Planck constant) are
disregarded.
We
show
here
that
all
phenomena
previously
attributed
to Einstein's relativity are in fact, the simple consequence
of application of the principle of mass-energy conservation in
atoms and molecules. There is no time dilation or space
contraction. There is only a change of clock rate and a
change of length of matter due to the change of Bohr
radius.
2
-Fundamental Mechanisms Inside Atoms.
The complex internal structure of atoms and molecules is
nothing but the sum of a few simple fundamental
relationships. The best way to verify the compatibility
between all these fundamental relationships and experimental
data is with simple atoms, in which each individual phenomenon
is recognizable independently. For example, it is well
known that all the deepest fundamental physics phenomena
involved in the structure of complex matter, appear under
their simplest fundamental form in atomic hydrogen.
Inside the hydrogen atom, we know that the Coulomb attracting
force between the two charged particles (electron and the
proton) is equal to the Newton centrifugal force of the
orbiting electron. This was recognized by Bohr. We
have:
 1
Where k is the Coulomb constant, e- is the electron charge, p+ is
the proton charge, m is the electron mass and r is the average
radius of the electron orbit, which is the Bohr radius.
The electron is orbiting the nucleus at a velocity ve. Furthermore, as discovered by de Broglie, the
basic principle of quantum mechanics is satisfied when the
circumference of the electron orbit is equal to an integer
number n of the de Broglie electron wavelength lB
. This relationship is explained by the Nobel Laureate G.
Herzberg in his book "Atomic Spectra and Atomic Structure"
(3). In atomic physics,
the parameter n is called the principal quantum number and r is
the radius of the electron orbit around the nucleus. This
gives:
1
Where k is the Coulomb constant, e- is the electron charge, p+ is
the proton charge, m is the electron mass and r is the average
radius of the electron orbit, which is the Bohr radius.
The electron is orbiting the nucleus at a velocity ve. Furthermore, as discovered by de Broglie, the
basic principle of quantum mechanics is satisfied when the
circumference of the electron orbit is equal to an integer
number n of the de Broglie electron wavelength lB
. This relationship is explained by the Nobel Laureate G.
Herzberg in his book "Atomic Spectra and Atomic Structure"
(3). In atomic physics,
the parameter n is called the principal quantum number and r is
the radius of the electron orbit around the nucleus. This
gives:
 and
and  2
The
lowest
(fundamental)
quantum
level
of
the
atom
is obtained, when the number of de Broglie electron wavelengths
n is equal to unity. A complete Rydberg series of quantum
levels is obtained when n equals the integers 1, 2, 3, . .
. This means that we can have 1, 2, 3 or more
integers of the de Broglie electron wavelengths in a complete
circumference of the electron orbit. For any of these
quantum numbers, we observe that the de Broglie electron wave is
always in phase, after each complete rotation. All the
atomic energy levels of hydrogen observed experimentally
correspond to the simultaneous application of relationships 1
and 2.
2
The
lowest
(fundamental)
quantum
level
of
the
atom
is obtained, when the number of de Broglie electron wavelengths
n is equal to unity. A complete Rydberg series of quantum
levels is obtained when n equals the integers 1, 2, 3, . .
. This means that we can have 1, 2, 3 or more
integers of the de Broglie electron wavelengths in a complete
circumference of the electron orbit. For any of these
quantum numbers, we observe that the de Broglie electron wave is
always in phase, after each complete rotation. All the
atomic energy levels of hydrogen observed experimentally
correspond to the simultaneous application of relationships 1
and 2.
Equations
1
and
2
are
in
a
perfect
agreement with experiments when the atom is stationary. It
is also an experimental fact that when an atom is in motion, the
same relationships are compatible within the moving atom, if
we use the relevant moving reference units [v].
However, we have seen previously (2) that in fact, the moving atoms are unquestionably
different, due to the absorbed kinetic energy, which gives an
extra mass to the electron and to the nucleus. The
resulting change of size of the reference units is due to the
increase of electron mass. These previously ignored
absolute physical modifications between frames are the origin of
the non-realistic relativity principle hypothesized by
Einstein. In order to take into account that both matter
and the size of the reference units are changing simultaneously
with velocity, we must note that a physical quantity cannot be
defined as a simple "number of reference units" as generally
used in papers. In contrast with a mathematical quantity,
we must define a physical quantity. A physical quantity
is an absolute quantity, defined as the product of the
number of units, multiplied by the size of the corresponding
reference unit.
Due
to
the
increase
of
mass
with
velocity,
the size of the reference unit is changing in different frames.
This variation of size of reference units must be taken into
account. We have seen previously (2), that we need a double index to get the relevant
information on the physical quantity being measured. For
example, we see that the number representing a mass m, can have
four different values, depending on the frame where it is
located and the reference unit used to measure it. We can
have ms[s], ms[v], mv[s] and mv[v]. The subscript after the physical quantity
refers to the frame where the particle is located. The
subscript s means that the particle is located in the stationary
frame, and the subscript v means that the particle is located in
the moving frame. Furthermore, the physical quantity is
also followed by a square parenthesis, which indicates the size
of the fundamental reference unit used to express that physical
quantity. The index [s] means that the corresponding
reference unit used is in the stationary frame. The index
[v] means that the reference unit used for that measurement is
in the moving frame.
We
will
see
that
there
exist
naturally
three
different situations when matter moves to a moving frame.
In most cases, an absolute physical quantity like a mass m, is
the product of the number of units (ms or mv) times the
size of the unit used to measure it, which is [s] or [v].
For example, an absolute physical length like rs[v] is the product of the number of units rs, times the size of the unit [v]. In the
second situation, the absolute size of the Coulomb and the
Planck constants must be considered when switching frames.
In the Coulomb case, nothing changes neither physically nor
mathematically. This is the case for the electric charges
e- and
protons p+. In that case, indexes are
irrelevant here, since the number of units and the size of units
are identical in all frames at any velocity. It is well
known that the absolute electric charge of electrons and protons
is constant when the particle is accelerated to high
velocity. This is an experimental fact, since
observational data have shown that, when an electron is
accelerated to a high velocity and then deflected in a magnetic
field, the electron "charge to mass ratio" (e/m) is changing in
a way, which is exactly compatible with the expected increase of
mass, assuming a constant electric charge and mass-energy
conservation. Consequently, we have:
 3
The
same
relationship
also
exists
for
the
proton.
The absolute electric charge of the positive proton is
independent of the velocity. This gives:
3
The
same
relationship
also
exists
for
the
proton.
The absolute electric charge of the positive proton is
independent of the velocity. This gives:
 4
We have seen previously (2)
that the electron mass increases g
times when the atom is accelerated to velocity va. This has been
demonstrated previously (see Web.) Due to the principle of
mass energy conservation, we have seen that the mass of all
bodies increases with velocity, following the increase of
kinetic energy. In that case, we have seen previously(2) that the mass of all particles like atoms, protons
and electrons increases according to:
4
We have seen previously (2)
that the electron mass increases g
times when the atom is accelerated to velocity va. This has been
demonstrated previously (see Web.) Due to the principle of
mass energy conservation, we have seen that the mass of all
bodies increases with velocity, following the increase of
kinetic energy. In that case, we have seen previously(2) that the mass of all particles like atoms, protons
and electrons increases according to:
 5
Where:
5
Where:
 6
In
equation
6,
v
is
the
absolute
velocity
of particles (electron, proton or atom), using stationary
units. Equations 5 and 6 only mean that when a particle or
even a macroscopic body is accelerated to a moving frame, there
is a real physical increase of mass due to the addition of the
external kinetic energy to that mass.
6
In
equation
6,
v
is
the
absolute
velocity
of particles (electron, proton or atom), using stationary
units. Equations 5 and 6 only mean that when a particle or
even a macroscopic body is accelerated to a moving frame, there
is a real physical increase of mass due to the addition of the
external kinetic energy to that mass.
In
physics,
the
parameters
in
equations
generally
represent
the number of standard units, (independently of
the size of the unit). It is arbitrarily assumed that the
size of the reference unit is constant in different
frames. However, it is not so when a moving observer is
moving with the mass, because the reference units in the moving
frame are different, due to their kinetic energy.
Then, the number (alone) of units, to measure
masses, lengths and clock rates is insufficient to represent a
physical quantity.
An
example
would
be
useful.
Let
us
consider
a rod having a length of 2.4 meters when measured in a
stationary frame with respect to a reference meter also in the
same frame. This length is written 2.4 ms[s]. If furthermore, that rod is carried to a
frame moving at velocity v and is measured with respect to the
reference meter also in the local frame (the moving frame), the
length of the same rod will be given as 2.4 mv[v]. We see that, in both cases, the length of
the rod is mathematically the same (i.e. 2.4 local
meters). However, the physical length of the rod is
certainly different in the moving frame. Also, the
physical length given by g times 2.4
ms[s] is equal to 2.4 mv[v]. Furthermore, the physical length 2.4
mv[s]
equals 2.4 mv[v]. The
reader must cautiously perceive the difference between the
mathematical equality and the physical equality.
Realistically, numbers are the only things
mathematical equations are calculating.
The
usual
equations
in
physics
completely
rely
on
an assumption of a definition of a reference unit, which is
assumed to be constant in any frame. This hypothesis is
erroneous. That hypothesis is not compatible with the
principle of mass-energy conservation (2).
The
parameters
in
a
normal
mathematical
equation
give
nothing but the number of units rather than the
size of the physical quantity. In previous papers (2,
4-7), the same number of units was instead
represented by the notation N-r, N-m and N-E.
3 - The Coulomb Energy Curve
Atom at Rest. - The
equilibrium between the centrifugal force and the electric
force between the electron and the nucleus is described in the
Bohr model. This is somewhat similar to the attracting
gravitational force between the orbiting planets around the
Sun as presented by Gerhard Herzberg (3) and others. When the atom is stationary,
equation 1 gives the relationship between the radius r of the
orbiting electron as a function of the orbiting velocity ve. From equation 1 we get:
 7
As
explained
above,
the
index
[s]
means
that
we are using the reference units existing in the stationary
frame. In a stationary frame, the term ke-p+/ms is a physical constant. Using equation 7
in classical physics, (but without quantum mechanics) the radius
of curvature r of the electron orbit is inversely proportional (
7
As
explained
above,
the
index
[s]
means
that
we are using the reference units existing in the stationary
frame. In a stationary frame, the term ke-p+/ms is a physical constant. Using equation 7
in classical physics, (but without quantum mechanics) the radius
of curvature r of the electron orbit is inversely proportional (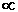 ) to the square of the
velocity. We have:
) to the square of the
velocity. We have:
 8
Equation
8
is
also
compatible
with
the
behavior
of the orbit of planets moving around the Sun described by
Newton and Kepler. It is found that the velocity of the
planets decreases as the square root of the distance from the
Sun. Similarly, in the case of the electron inside the
atom, the electrical potential energy E of the electron
corresponding to equation 1 is:
8
Equation
8
is
also
compatible
with
the
behavior
of the orbit of planets moving around the Sun described by
Newton and Kepler. It is found that the velocity of the
planets decreases as the square root of the distance from the
Sun. Similarly, in the case of the electron inside the
atom, the electrical potential energy E of the electron
corresponding to equation 1 is:
 9
In
equation
9,
we
see
that
the
radius
r of the electron orbit can vary continuously, as long as the
quantization of quantum mechanics is ignored. However, in
the microscopic world of quantum mechanics, there is another
constraint given by equation 2, which requires that the
circumference of the electron orbit be equal to an integer
number of de Broglie wavelengths. This is the fundamental
mechanism of quantization. The Newton and Coulomb
equations above must always be satisfied in quantum mechanics,
but there is a further essential requirement given by the de
Broglie equation, due to the wave nature of matter.
This de Broglie constraint will be calculated below. Let
us simplify the notation using only v instead of ve for the electron velocity.
9
In
equation
9,
we
see
that
the
radius
r of the electron orbit can vary continuously, as long as the
quantization of quantum mechanics is ignored. However, in
the microscopic world of quantum mechanics, there is another
constraint given by equation 2, which requires that the
circumference of the electron orbit be equal to an integer
number of de Broglie wavelengths. This is the fundamental
mechanism of quantization. The Newton and Coulomb
equations above must always be satisfied in quantum mechanics,
but there is a further essential requirement given by the de
Broglie equation, due to the wave nature of matter.
This de Broglie constraint will be calculated below. Let
us simplify the notation using only v instead of ve for the electron velocity.
Atom
in Motion. - When an atom is in motion, due to
the kinetic energy transferred from the external frame, (which
is added to the particle), the principle of mass-energy
conservation requires that the mass of all particles
increases. Therefore, as given by equation 5, the electron
mass increases inside the atom, due to the higher velocity of
the atom, just as the proton mass. Let us calculate the
change of the electron orbital velocity inside the atom, and
around the nucleus, due to the increase of electron mass (from ms[s] to gms[s]). Substituting gms[s] in equation 7 gives:
 10
In
equation
10,
we
have
assumed
momentarily
that
the Bohr radius remains rs[s].
We will see below that this is not compatible with the de
Broglie equation. We have seen previously (2) that in order to satisfy the principle of mass-energy
conservation, the size of the Bohr radius must necessarily
increase g times as a
function of the velocity of the atom. This is in perfect
agreement with the calculations and observations (2). This increase of the Bohr radius is tested
here. This absolute increase of size of the Bohr
radius, as a function of the velocity of the atom is:
10
In
equation
10,
we
have
assumed
momentarily
that
the Bohr radius remains rs[s].
We will see below that this is not compatible with the de
Broglie equation. We have seen previously (2) that in order to satisfy the principle of mass-energy
conservation, the size of the Bohr radius must necessarily
increase g times as a
function of the velocity of the atom. This is in perfect
agreement with the calculations and observations (2). This increase of the Bohr radius is tested
here. This absolute increase of size of the Bohr
radius, as a function of the velocity of the atom is:
 11
In
that
case,
let
us
calculate
the
electron
velocity vv in the moving
atom, (when the Bohr radius is grs[s]). This increase of rs with equation 10 gives:
11
In
that
case,
let
us
calculate
the
electron
velocity vv in the moving
atom, (when the Bohr radius is grs[s]). This increase of rs with equation 10 gives:
 12
In
equation
12,
the
physical
transformations
related
to
the motion of the atom are taken into account. However, we
recall that we are still using the [s] reference units.
Let us calculate now the relative electron velocity around the
nucleus for the stationary atom (equation 7), with the
corresponding electron velocity due to the increase of electron
mass and of the Bohr radius. Equations 7 and 12 give:
12
In
equation
12,
the
physical
transformations
related
to
the motion of the atom are taken into account. However, we
recall that we are still using the [s] reference units.
Let us calculate now the relative electron velocity around the
nucleus for the stationary atom (equation 7), with the
corresponding electron velocity due to the increase of electron
mass and of the Bohr radius. Equations 7 and 12 give:
 13
Equation 13, also gives:
13
Equation 13, also gives:
 14
Equation
14
shows
that
when
the
atom
is
accelerated to velocity v, the electron velocity around the
nucleus is reduced g
times. Of course, we have seen previously that the size of
the reference unit "velocity" is the same in all frames.
From equations 11 and 14 we have:
14
Equation
14
shows
that
when
the
atom
is
accelerated to velocity v, the electron velocity around the
nucleus is reduced g
times. Of course, we have seen previously that the size of
the reference unit "velocity" is the same in all frames.
From equations 11 and 14 we have:
 15
As
a
consequence
of
an
increase
of
atom
velocity (not the increase of electron velocity inside the
atom), equation 15 means that the electron velocity inside the
atom varies as the inverse of the radius of the electron
orbit. Using the same notation as in equation 8, equation
15 gives:
15
As
a
consequence
of
an
increase
of
atom
velocity (not the increase of electron velocity inside the
atom), equation 15 means that the electron velocity inside the
atom varies as the inverse of the radius of the electron
orbit. Using the same notation as in equation 8, equation
15 gives:
 16
We
must
notice
the
difference
between
equations
16
and 8. In equation 8, we are changing directly the
electron velocity inside an atom. In equation 16, the
change of electron energy is indirect, because it is the
consequence of the change of velocity of the atom. In
order to picture more clearly the consequence of the increase of
distance between the electron and the nucleus due to the
increase of atom velocity, let us represent on figure 1, the
Coulomb potential as a function of the distance between the
electron and the nucleus.
16
We
must
notice
the
difference
between
equations
16
and 8. In equation 8, we are changing directly the
electron velocity inside an atom. In equation 16, the
change of electron energy is indirect, because it is the
consequence of the change of velocity of the atom. In
order to picture more clearly the consequence of the increase of
distance between the electron and the nucleus due to the
increase of atom velocity, let us represent on figure 1, the
Coulomb potential as a function of the distance between the
electron and the nucleus.

Figure 1
On
figure
1,
the
two
heavy
curves
represent
the Coulomb potential given in equation 1. The orbit
(ellipse) As represents the
electron orbit of the stationary hydrogen atom in its ground
quantum state (ns=1) where n
is the principal quantum number. The radius of this orbit
is rs. Of course, other
quantum states like Bs (ns=2) and other states above, exist at higher electron
energy in the Coulomb curve in agreement with equation 2 when
the atom velocity is zero. When the atom is accelerated to
high velocity, the electron mass becomes larger, so that the
series of quantum states As, Bsand the other states above, are shifted to the series
of quantum states Av, Bv etc... . The Bohr radii of all the
corresponding quantum states are shifted to a larger
radius. The atom in motion becomes in the quantum states nv=1, 2, 3 etc... as illustrated on figure 1.
4 - Physical Transformations.
When
the
atom
moves
from
the
[s]
frame
to the [v] frame, we have seen that there is an increase of the
Bohr radius, which increases physically from rs[s] to grs[s] (which is physically equal to rv[v]) . Due to this increase of radius (equation
11) there is a decrease of energy in the atom that leads to the
energy Ev[s] in the moving
atom. Using [s] units, the electrostatic energy between
the electron and the proton is:
 17
In
equation
17,
we
see
that
when
the
atom is located in the [v] frame and using the [v] reference
units, using classical physics, we get the same mathematical
relationship as the one using the stationary units, with the
atom in the stationary frame. However, apart from the
mathematical relationship, which is the same when the atom is
transferred to the moving frame, there is one physical change
that took place during these transformations. When
calculating the Bohr radius, the same mathematical relationship
does not mean the same absolute energy. Due to the
transfer of the atom to the moving frame, the Bohr radius
increases from rs to grs.
The dilated atom is then in the moving frame. Let us
calculate the absolute energy of that atom. Substituting
the Bohr radius rs for grs in
equation 9, we see that the absolute energy Ev[s] is g times smaller,
when the atom is in the moving frame. This gives:
17
In
equation
17,
we
see
that
when
the
atom is located in the [v] frame and using the [v] reference
units, using classical physics, we get the same mathematical
relationship as the one using the stationary units, with the
atom in the stationary frame. However, apart from the
mathematical relationship, which is the same when the atom is
transferred to the moving frame, there is one physical change
that took place during these transformations. When
calculating the Bohr radius, the same mathematical relationship
does not mean the same absolute energy. Due to the
transfer of the atom to the moving frame, the Bohr radius
increases from rs to grs.
The dilated atom is then in the moving frame. Let us
calculate the absolute energy of that atom. Substituting
the Bohr radius rs for grs in
equation 9, we see that the absolute energy Ev[s] is g times smaller,
when the atom is in the moving frame. This gives:
 18
In
order
to
be
able
to
compare
the
internal energy inside the atom when it moves from the
stationary frame to the moving frame, equation 18 is claculated
using the same reference [s] units. Equation 18 shows that
the absolute amount of energy available in quantum transitions
is also smaller in the moving frame, although the mathematical
relationship is the same. This is in perfect
agreement with all experimental data, since it is also an
experimental fact that the quantum transitions emitted by moving
atoms possesses a smaller amount of energy. Therefore the
moving observer must utilize the same classical mathematical
relationships when he uses the units existing in his moving
frame. We must conclude that the observer in any moving
frame will always get the correct answer when using the same
mathematical relationship, but the use of the proper units leads
to a different absolute energy.
18
In
order
to
be
able
to
compare
the
internal energy inside the atom when it moves from the
stationary frame to the moving frame, equation 18 is claculated
using the same reference [s] units. Equation 18 shows that
the absolute amount of energy available in quantum transitions
is also smaller in the moving frame, although the mathematical
relationship is the same. This is in perfect
agreement with all experimental data, since it is also an
experimental fact that the quantum transitions emitted by moving
atoms possesses a smaller amount of energy. Therefore the
moving observer must utilize the same classical mathematical
relationships when he uses the units existing in his moving
frame. We must conclude that the observer in any moving
frame will always get the correct answer when using the same
mathematical relationship, but the use of the proper units leads
to a different absolute energy.
This
result
is
not
compatible
with
the
Einstein's
invariance principle, because even if the same equations are
valid, they do not represent the same absolute amount of
energy. All the quantum transitions appear
the same internally, in all frames, because the size of the
reference units changes in a way that compensate exactly for the
change of size of the physical quantities. Furthermore,
the explanation here is compatible with a physical reality,
independent of the observer, contrary to Einstein's
relativity. There is no time dilation, no space
contraction. It is a simple moving atom, which naturally
will emit a lower absolute frequency as observed experimentally.
5 - Quantum Conditions - De Broglie
Wavelength.
We
have
seen
that
inside
a
stationary
atom,
as well as inside a moving atom, the Newton's centrifugal
force on the orbiting electron is equal and in the opposite
direction to the Coulomb force. We have shown above that
this requirement is perfectly satisfied inside the atom when
we use the stationary units when the atom is stationary, and
when we use the moving units when the atom is moving.
This is done logically without using the Einstein's relativity
hypothesis.
However,
there
is
another
condition
that
needs
to
be verified to be compatible with quantum mechanics. We
have seen in equation 2, that due to quantum mechanics, the
atom must be compatible with the de Broglie equation. This
condition is fundamental and corresponds to the quantization
of electron energy in atoms. This quantum condition
requires that the circumference of the electron orbit is equal
to an integer n, times the de Broglie electron wavelength lB.
In atomic physics, this integer is called the principal
quantum number. Let us consider the lowest
principal quantum number (when n equals unity). We can
show also that all other quantum numbers (for n=2, 3, 4, etc.)
satisfy the solution presented here. According to de
Broglie, the circumference of the lowest electron orbit in an
atom in a stationary frame must be:
 19
Since
it
is
an
experimental
fact
that
we
can always apply successfully the same equation in all frames,
we need to show that equation 19 must also be valid for a moving
atom, when we use moving reference units. We show here
that the phenomenon is also compatible with the equation that
uses the moving units which gives:
19
Since
it
is
an
experimental
fact
that
we
can always apply successfully the same equation in all frames,
we need to show that equation 19 must also be valid for a moving
atom, when we use moving reference units. We show here
that the phenomenon is also compatible with the equation that
uses the moving units which gives:
 20
Since
the
Planck
constant
"h"
possesses
its
own
units, (it is not a pure number as p),
we will see now that the change of mass and length units
(required between frames due to mass-energy conservation), is
responsible for the change of size of the reference Planck unit
in the moving frame. We use a dimensional analysis to
calculate that change of size of units. Let us formulate a
dimension analysis, based on the well-known energy-frequency
relationship giving the energy of a photon.
20
Since
the
Planck
constant
"h"
possesses
its
own
units, (it is not a pure number as p),
we will see now that the change of mass and length units
(required between frames due to mass-energy conservation), is
responsible for the change of size of the reference Planck unit
in the moving frame. We use a dimensional analysis to
calculate that change of size of units. Let us formulate a
dimension analysis, based on the well-known energy-frequency
relationship giving the energy of a photon.
 21
Where n[s] is the frequency of the
photon measured using a clock located in the stationary
frame. In the moving frame, using the [v] units, in order
to be coherent, the relationship corresponding to equation 21
must be:
21
Where n[s] is the frequency of the
photon measured using a clock located in the stationary
frame. In the moving frame, using the [v] units, in order
to be coherent, the relationship corresponding to equation 21
must be:
 22
By definition, the frequency n means
the number of cycles per local second, which is defined as the
difference of clock display on the local clock. That local
clock is an atomic clock, which counts the number of cycles
emitted by a local atom during a time interval. In
physics, a unit of time interval is defined as the time interval
during which a standard number of cycles is emitted by an atomic
clock. Consequently, when both an atom to be measured, and
also a standard clock are simultaneously moved to a frame at
velocity v, the quantum emission rate of the atom to be
investigated, as well as the quantum emission rate of the atom
which determines the atomic local time, will both vary by the
same ratio (g). Consequently,
for any velocity of that frame, the frequency of the atom being
studied using the local clock will always be the same.
They both change the same way as given in quantum
mechanics. Consequently, the local frequency is the same
in all moving frames when measured with local units. This gives:
ns[s]=nv[v]= n
23
We
must
also
calculate,
what
is
value
of
the Planck constant h[v] in the moving frame. In order to
calculate the size of the reference Planck constant units
existing in different frames, we compare the Planck equations 21
and 22. Since we consider an absolute amount of energy,
the sub index is then useless (always s). Combining
equation 23 with 22 and 21 gives:
22
By definition, the frequency n means
the number of cycles per local second, which is defined as the
difference of clock display on the local clock. That local
clock is an atomic clock, which counts the number of cycles
emitted by a local atom during a time interval. In
physics, a unit of time interval is defined as the time interval
during which a standard number of cycles is emitted by an atomic
clock. Consequently, when both an atom to be measured, and
also a standard clock are simultaneously moved to a frame at
velocity v, the quantum emission rate of the atom to be
investigated, as well as the quantum emission rate of the atom
which determines the atomic local time, will both vary by the
same ratio (g). Consequently,
for any velocity of that frame, the frequency of the atom being
studied using the local clock will always be the same.
They both change the same way as given in quantum
mechanics. Consequently, the local frequency is the same
in all moving frames when measured with local units. This gives:
ns[s]=nv[v]= n
23
We
must
also
calculate,
what
is
value
of
the Planck constant h[v] in the moving frame. In order to
calculate the size of the reference Planck constant units
existing in different frames, we compare the Planck equations 21
and 22. Since we consider an absolute amount of energy,
the sub index is then useless (always s). Combining
equation 23 with 22 and 21 gives:
 24
Also,
we
have
seen
in
equation
5
that
in the moving frame, the size of unit of mass is g times larger than in the size of same
mass when it was in the rest frame. Since there is
complete equality between mass and energy, the same relationship
holds for energy as for mass. Therefore a size of
the unit of mass is g times larger
in the moving frame, just as the size of the unit of energy is g times larger in the moving frame,
because they are both related by the constant c2. Therefore, as seen in equation 5, the relative
physical size of the reference units of energy is:
24
Also,
we
have
seen
in
equation
5
that
in the moving frame, the size of unit of mass is g times larger than in the size of same
mass when it was in the rest frame. Since there is
complete equality between mass and energy, the same relationship
holds for energy as for mass. Therefore a size of
the unit of mass is g times larger
in the moving frame, just as the size of the unit of energy is g times larger in the moving frame,
because they are both related by the constant c2. Therefore, as seen in equation 5, the relative
physical size of the reference units of energy is:
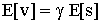 25
Equation
25
has
nothing
in
common
with
equation
18. We have seen that equation 18 is related to the
electron velocity inside the atom. It gives the change of
energy inside the hydrogen atom due to the change of electron
velocity, which brings equilibrium between the electronic force
and the centrifugal force. In equation 25, it is the atom
velocity, which is involved (not the internal electron).
Equation 25 gives the relative change of local units of energy,
due to the important change of velocity of the atom in a moving
frame of reference. Substituting equation 25 in 24 gives:
25
Equation
25
has
nothing
in
common
with
equation
18. We have seen that equation 18 is related to the
electron velocity inside the atom. It gives the change of
energy inside the hydrogen atom due to the change of electron
velocity, which brings equilibrium between the electronic force
and the centrifugal force. In equation 25, it is the atom
velocity, which is involved (not the internal electron).
Equation 25 gives the relative change of local units of energy,
due to the important change of velocity of the atom in a moving
frame of reference. Substituting equation 25 in 24 gives:
 26
which gives
26
which gives
 27
Equation
27
gives
the
relative
size
of
the
reference units of the Planck energy constant between the rest
and the moving frame. Similarly to the increase of energy
(or the increase of mass) in the moving frame, equation 27 shows
that the size of the Planck constant is g times
larger in the moving frame. Of course, the number
of units giving the Planck constant remains the same in all
frames.
27
Equation
27
gives
the
relative
size
of
the
reference units of the Planck energy constant between the rest
and the moving frame. Similarly to the increase of energy
(or the increase of mass) in the moving frame, equation 27 shows
that the size of the Planck constant is g times
larger in the moving frame. Of course, the number
of units giving the Planck constant remains the same in all
frames.
6 - Testing the de Broglie Equation.
Let
us
consider
the
de
Broglie
relationship.
When
we consider an atom at rest, we know that the electron
wavelength in the ground state of the atom is equal to the de
Broglie electron wavelength. Let us verify now, that the
moving atom calculated above is compatible with de Broglie
equation, even when we use the [v] reference units.
Since this is an experimental fact that the same de Broglie
equation is valid in all frames, we need to show that equation
19 must also be valid in a moving atom, when we use moving
reference units. In the stationary frame equation 19 is:
 28
In the moving frame we must have:
28
In the moving frame we must have:
 29
Starting
with
a
normal
atom
in
the
stationary
[s] frame, we calculate the same atom, substituting the
transformations resulting from mass-energy conservation
calculated above. In a first stage, we make the physical
transformations expected to follow from the principle of
mass-energy conservation as seen by an observer at rest.
In a second step, we will express the same physical quantities
using the moving units instead of the stationary units as seen
in the moving frame.
29
Starting
with
a
normal
atom
in
the
stationary
[s] frame, we calculate the same atom, substituting the
transformations resulting from mass-energy conservation
calculated above. In a first stage, we make the physical
transformations expected to follow from the principle of
mass-energy conservation as seen by an observer at rest.
In a second step, we will express the same physical quantities
using the moving units instead of the stationary units as seen
in the moving frame.
Physical
Transformations.
With
respect
to
initial
conditions,
we
have
the
following transformations. From equation 11, we see that
the Bohr radius increases according to:
 30
Also,
due
to
the
velocity,
(see
equation
5),
the mass increases according to:
30
Also,
due
to
the
velocity,
(see
equation
5),
the mass increases according to:
 31
Also,
due
to
the
increase
of
atom
velocity,
we see from equation 14, that the electron velocity inside the
atom decreases according to:
31
Also,
due
to
the
increase
of
atom
velocity,
we see from equation 14, that the electron velocity inside the
atom decreases according to:
 32
Local Moving Units.
32
Local Moving Units.
We
have
seen
in
equation
27
that
using
the same numerical Planck constant, but due to the increase of
size of the units in the moving frame, the Planck physical
quantity in the moving frame is g
times larger (just as for energy in that frame). For the
observer at rest, the larger physical Planck constant (in
motion) must be substituted. Equation 27 gives:
 33
Substituting
equations
30,
31,
32,
and
33
in
equation 28 gives:
33
Substituting
equations
30,
31,
32,
and
33
in
equation 28 gives:
 34
Equation
34
gives
the
consequences
of
mass-energy
conservation
to atoms. Let us express the same physical length grs[s]
of the Bohr radius but using the longer moving reference
units. This gives:
34
Equation
34
gives
the
consequences
of
mass-energy
conservation
to atoms. Let us express the same physical length grs[s]
of the Bohr radius but using the longer moving reference
units. This gives:
 35
Similarly,
when
the
increased
mass
is
measured
using
the larger reference units in the moving frame, we get:
35
Similarly,
when
the
increased
mass
is
measured
using
the larger reference units in the moving frame, we get:
 36
Also,
we
have
seen
that
the
electron
velocity
is g times slower in the moving
frame. In agreement with the description of the moving
atom, the electron velocity in the moving frame is g times slower. This gives:
36
Also,
we
have
seen
that
the
electron
velocity
is g times slower in the moving
frame. In agreement with the description of the moving
atom, the electron velocity in the moving frame is g times slower. This gives:
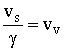 37
Finally,
due
to
the
change
of
units
in
the moving frame, we have seen in equation 27 that the Planck
constant is different in the moving frame according to:
37
Finally,
due
to
the
change
of
units
in
the moving frame, we have seen in equation 27 that the Planck
constant is different in the moving frame according to:
 38
Substituting
equations
35,
36,
37
and
38
in
equation 34 give:
38
Substituting
equations
35,
36,
37
and
38
in
equation 34 give:
 39
Equation
39
gives
the
de
Broglie
relationship
calculated
using only the principle of mass-energy, quantum mechanics and
classical physics. We see that the relationship 39 is
identical to equation 29. This shows that the moving
observer measuring the structure of his own atoms in his frame
will find the same fundamental equations as in quantum mechanics
without using any of the Einstein's principle of
relativity. This appears naturally using only conventional
logic and mass-energy conservation.
39
Equation
39
gives
the
de
Broglie
relationship
calculated
using only the principle of mass-energy, quantum mechanics and
classical physics. We see that the relationship 39 is
identical to equation 29. This shows that the moving
observer measuring the structure of his own atoms in his frame
will find the same fundamental equations as in quantum mechanics
without using any of the Einstein's principle of
relativity. This appears naturally using only conventional
logic and mass-energy conservation.
7 - Observations and Discussions.
We
must
conclude
that
the
moving
observer
gets
the correct physical predictions when he uses the same
equation as the stationary observer. The requirement is
that the moving observer must use the moving units and the
stationary observer must use the stationary units. This
may appear equivalent to Einstein's principle, which claims
that nothing is changed after the acceleration of the
frame. However, Einstein's hypothesis is erroneous,
because in fact, the electron velocity, the Bohr radius and
the masses of particles are modified. Einstein did not
realize that simple logic and the principle of mass-energy
conservation leads to a modification of the reference units in
the moving frame that compensates exactly for the real
physical changes taking place when masses are
accelerated. Space-time distortions are useless and
non-realistic. Einstein's principle of invariance is an
error, because we cannot claim a real invariance in physics
when the atoms in different frames have a different Bohr
radius, a different electron mass and also emit different
frequencies. As explained in this paper, a numerical
invariance is unsatisfactory in physics, because the size of
the units inevitably changes between frames. The
physical changes are just perfectly compensated by an
equivalent change of the size of the moving reference units of
mass, length and clock rate in different frames. Similarly in
the Lorentz transformations, nothing takes into account that
the size of the units are compelled to change. Lorentz
did not realize that a constant number of units between all
frames could simply be explained by the change of size of
these units following the principle of mass-energy
conservation. Nature has made the laws of physics so
that they appear undistinguishable internally, but this
difference can be measured from an external location.
The
above
results
also
imply
that
there
is
an absolute frame of reference for light so that the velocity
of light is (c+v) and (c-v) as confirmed experimentally using
the GPS(4). The GPS
system (just as the Sagnac effect) provides a striking proof
of an absolute frame of reference for light propagation, but
conservatism and non-realism prevent scientists from accepting
that evidence. We must also realize that the change of
clock rate and the increase of length of matter is an
observational fact. We can see that the slowing down of
moving atomic clocks with velocity requires necessarily an
increase of the Bohr radius and therefore an increase of size
of matter as shown here. When we apply Newton
mechanics with these classical transformations of length and
clock rate to calculate the advance of the perihelion of
Mercury, we have shown(5)
that they lead naturally to the observed advance, without
having to assume the magic of relativity. This result
agrees even with the assumed deflection of light by the Sun(6). The present analysis also implies that
a positive result is expected from the kind of measurements
known as the Michelson-Morley experiment. A recent
thorough study(7) of these
data has shown that it is so. Munera's analysis(7) shows that an unbiased analysis reveals different
kind of errors, which reveal that there is indeed a shift in
optical fringes in the Michelson-Morley type of experiment
that has been overlooked previously.
This
velocity
dependent
variation
of
the
internal
electronic
structure of atoms, does not only exist in the electron
shells, but also exists in the nuclear structure. Since
the Planck constant and masses and other fundamental constants
are also involved in the nucleus of matter, we can calculate
now, the change in the nuclear structure and nuclear forces as
a function of the velocity of those nuclei. Therefore,
the change of lifetimes of radioactive nuclei is predictable
without Einstein's relativity principles, using the same
principle of mass-energy conservation as above. In the
same context, we see that this paper agree with Terrel(8) who found that length contractions and dilations
are not measurable to the observer in the moving frame.
This work has also some similarities with the work of Ives(9) who also used energy and momentum
conservation. Also Munera(10) found an increase of mass in a gravitational and in
an electric field.
Finally,
it
is
important
to
realize
that
a
similar modification of the structure of atoms can be
calculated when the energy of the electron inside the atom is
perturbed due to gravitational potential, instead of kinetic
energy as calculated here. There has been a calculation
of that effect previously(2),
but the complete detailed explanation will be published in a
coming paper. We will see that the change of
gravitational energy can explain logically all the phenomena
previously requiring the Einstein's hypotheses.
8 - References.
(1) A. Einstein, Die Grundlage der allgemeine
Relativitatstheorie, Ann. Phys. 49, 769-822
(1916).
(2)
P. Marmet, Einstein's Theory of Relativity versus
Classical Mechanics, Newton Physics Books, Ogilvie
Road Gloucester On. Canada pp. 200, ISBN 0-921272-18-9
(1997).
(3)
G. Herzberg, Atomic Spectra and
Atomic Structure Dover Publications, New
York, pp. 258. 1944.
(4)
P. Marmet Explaining the Illusion of the Constant
Velocity of Light, Meeting "Physical
Interpretations of Relativity Theory VII" University of
Sunderland, London U.K., 15-18, September 2000. Conference
Proceedings "Physical Interpretations of Relativity Theory
VII" p. 250-260 (Ed. M. C. Duffy, University of
Sunderland). Also in Acta Scientiarum (2000): The
GPS and the Constant Velocity of Light. Also
presented at NPA Meeting University of Conn, Storrs in June
2000. Also submitted for publication in Galilean
Electrodynamics in 2000. On the Web at: https://www.newtonphysics.on.ca/illusion/index.html
(5)
P. Marmet, Classical Description of the Advance
of the Perihelion of Mercury, Physics Essays, Volume 12,
No: 3, 1999, P. 468-487. Also, P. Marmet, A Logical and
Understandable Explanation to the Advance of the
Perihelion of Mercury, invited speaker Society for
Scientific Exploration, Albuquerque, June 3-5, 1999. Also on
the Web: A Detailed Classical Description of the
Advance of the Perihelion of Mercury. at the
address:
https://www.newtonphysics.on.ca/mercury/index.html
(6)
P. Marmet and C. Couture, Relativistic Deflection
of Light Near the Sun Using Radio Signals and Visible
Light, Physics Department, University of
Ottawa, Ottawa, On. Canada, K1N 6N5 Physics Essays Vol:
12, No: 1 March 1999. P. 162-174. Also
The Deficient Observations of Light Deflection Near the
Sun NPA Meeting University of Conn, Storrs in June
2000. Also on the USB Drive: Relativistic
Deflection of Light Near the Sun Using Radio Signals and
Visible Light at: newtonphysics.on.ca/eclipse/index.html
(7)
Héctor Múnera, Michelson-Morley Experiments
Revisited: Systematic Errors, Consistency Among Different
Experiments, and Compatibility with Absolute Space,
Apeiron, Vol. 5 Nr. 1-2, January-April 1998.
(8) L.
Terrel, Phys. Rev. Vol. 116, 1041 (1959)
(9) H. E.
Ives, Philos Mag. Series 7, Vol. 36, 392-403 (1945)
(10) H.
A. Múnera, A Quantitative Formulation of Newton’s
First Law, Physics Essays, Vol. 6,
173-180 (1993)
-------------------------------------------------
Invited paper, Journal of New
Energy, ISSN 1086-8259, Vol. 6, No: 3, pp. 103-115,
Winter 2002.
----------------------------------------------------------------------------------------------------------
Return to: Top of Page
Return to: List of
Papers on the Web
About
the Author
July 9, Nov, 2001
 1
1 1
1 and
and  6
6 7
7 8
8 9
9 10
10 12
12 13
13 14
14 15
15 16
16
 17
17 19
19 20
20 24
24 26
26 28
28 29
29 34
34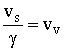 37
37